-
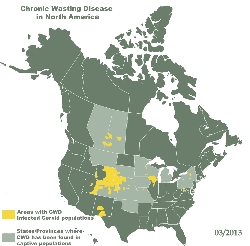
Chronic Wasting
Disease or CWD, is a progressive, degenerative disease of the brain
affecting deer and elk (Cervidae) in
North America.
-
CWD
is similar to other nervous system diseases known as transmissible
spongiform encephalopathies (TSE). These include scrapie in sheep,
bovine spongiform encephalopathy (BSE) in cattle, and
Creutzfeldt-Jakob disease (CJD) in humans. Although the exact cause
of CWD is unknown, it is associated with the presence of an abnormal
protein called a prion. There is no treatment or vaccine currently
available for the disease.
-
There is currently no scientific evidence that CWD affects humans,
but we must exercise caution since there is some evidence to suggest
that BSE, or “mad
cow disease”,
can affect humans.
-
It
is not clear how CWD is transmitted but, based on experience with
the disease in captive deer, both animal to animal and mother to
offspring transmission may be possible; however, the most likely
means of transmission is between animals that are in close contact
with each other. In addition, elk and mule deer became infected
after being placed in paddocks that had previously housed infected
cervids, even though there were no other
cervids
presently on
the premises, leading to the assumption that the agent could survive
in the environment and cause disease.
-
Symptoms of infected animals may include lack of coordination,
separation from other animals in a herd, excess salivation,
depression, unusual behavior,
paralysis, weight loss, difficulty swallowing, increased thirst
and urination, and pneumonia. Signs usually last for weeks to months
before the animal dies; however, some animals may not show
clinical signs except for an
acute
pneumonia. Animals are usually 3- to 4-years of age before
clinical signs appear, but may be as young as 18 months or as old as
13 years. The disease is tentatively diagnosed based on
clinical signs, and is confirmed by isolating abnormal prion proteins
during
postmortem examination of the brain stem or from samples of
lymphoid
tissue from the affected animal. Current research, however, suggests
that biopsies of palatine tonsils or rectal lymphoid tissue could be
used to determine the presence of prion proteins in live animals.
|