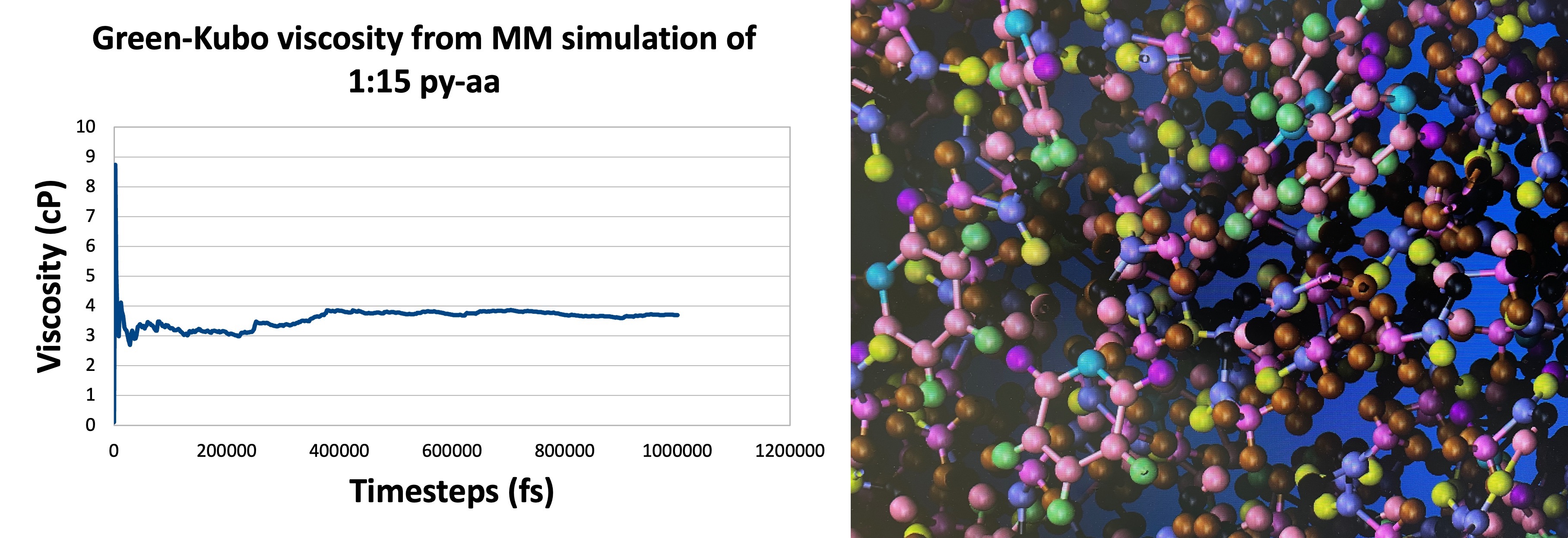
Ionic liquids (ILs) are interesting towards electrochemical applications due to their intermediate-high electrical conductivity, workable viscosity, low volatility, and high thermal stability. Studies on pyridine - acetic acid system (a simple protic ionic liquid (PIL)) using ab initio molecular dynamics (AIMD) by East's research group have shown that the electrical conductivity mechanism in this system is through translation of ions and NOT Grotthuss type. They were able to explain the maximum in conductivity (vs Temperature) observed in this system by developing a kite-model based on the liquid structure observed in the simulations. Our group is using molecular mechanics (MM) to simulate the traditional pyridine - acetic acid (py-aa) system and similar PIL systems to further substantiate the kite model proposed by East group and arrive at a standard method/model for prediction of transport properties of Ionic Liquids. So far we have successfully reproduced the viscosity maximum (vs Temperature) in py-aa using MM within a 2.5 cP error bar and we are in the process of reproducing the conductivity maximum by calculating the mobility of ions in the system. To address a possible effect of delta(pKa) on the position of the maximum relative to other PILs, we are doing similar studies on pyridine - trifluoro acetic acid (py-faa) system as well.

There are several other parallel projects running in the group, the details of which will be updated here when progress is made. So, stay tuned!!!